Electric Cells and Batteries
📚 Key Concepts
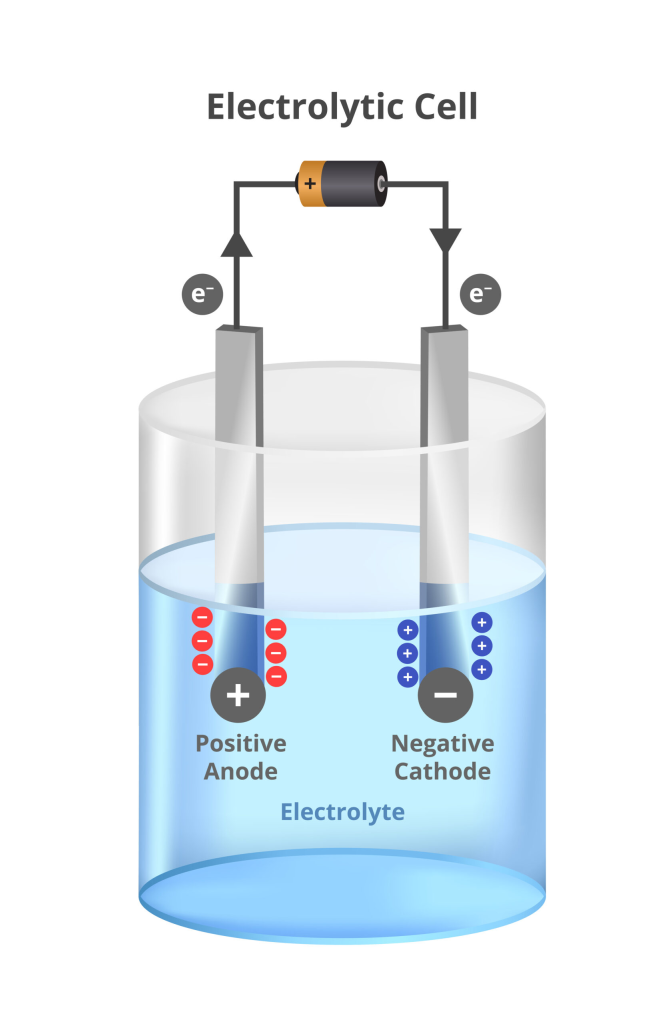
🔹 How Batteries Work

A battery generates electric current due to chemical reactions taking place inside it.
Basic Components:
- Electrodes – Two metal plates of different materials
- Electrolyte – Chemical solution that conducts electricity
- Container – Houses the electrodes and electrolyte
- Terminals – Positive and negative connection points
🔹 Types of Electric Cells
1. Voltaic/Galvanic Cell:
- Historical significance – First electric cell invented
- Construction – Two different metal plates in electrolyte
- Working – Chemical reaction produces electricity
- Limitation – Single use, liquid electrolyte
2. Dry Cell:
- Modern design – Most widely used today
- Electrolyte – Thick moist paste (not liquid)
- Construction – Zinc container + Carbon rod + Paste
- Advantage – Portable, leak-proof
- Limitation – Single use, must be disposed after use
3. Rechargeable Batteries:
- Reusable – Can be charged and used multiple times
- Cost-effective – Saves money over time
- Environmental – Reduces waste
- Applications – Phones, laptops, vehicles, inverters
- Limitation – Eventually wear out after many cycles
🔹 Battery Chemistry
Lithium-ion Batteries:
- Most common rechargeable battery type
- High energy density – Store more power in smaller size
- Materials – Lithium, cobalt, and other special metals
- Future – Solid-state batteries being developed
Metal Electrode Pairs:
- Zinc/Copper, Iron/Copper, Aluminum/Copper
- Different metals have different electrical properties
- Some act as positive, others as negative electrodes
🧪 Practical Activity
🔸 Lemon Battery: Copper wire + Iron nail + Lemon juice = Electric current!
🔸 LED Test: Use multiple lemons in series to light up LED
