pH Scale – Measuring Acidity and Basicity
📚 Key Concepts
🔹 Real-Life Example
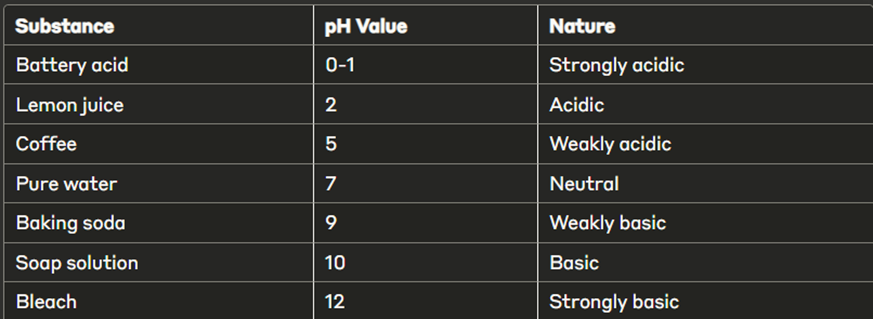
Think of pH as a thermometer for acids and bases! Just as a thermometer measures how hot or cold something is, pH measures how acidic or basic a solution is. Pure water has pH 7 (neutral), lemon juice has pH 2 (very acidic), and soap solution has pH 10 (basic).
pH Scale: A scale that measures the hydrogen ion concentration in a solution, ranging from 0 to 14. pH stands for “power of hydrogen” or “potential of hydrogen.”

🧪 pH Scale Understanding
🔸 pH Values and Nature
- pH < 7: Acidic solutions (more H⁺ ions)
- pH = 7: Neutral solutions (equal H⁺ and OH⁻ ions)
- pH > 7: Basic solutions (more OH⁻ ions)
🔸 Common pH Values
🔍 Advanced: pH in Daily Life
- Human blood: pH 7.4 (slightly basic)
- Stomach acid: pH 1.5-2.0 (highly acidic for digestion)
- Skin: pH 4.5-6.0 (slightly acidic for protection)
- Soil: pH 6.0-7.5 (optimal for most plants)
